Abstract
Background:
Genomic evaluation of structural and molecular alterations is essential for the classification and risk stratification of patients with acute myeloid leukemia (AML). Cytogenetics (CG) has traditionally been used for the identification of large structural or copy number changes and molecular studies for small sequence changes. Rapid technical advances and decreased sequencing (seq) costs have made whole-genome sequencing (WGS) more feasible and recent efforts have suggested it can match the analytic performance of traditional CG while providing additional prognostic information. However, the use of WGS in the clinic is still in its infancy and questions remain regarding the utility of many factors including seq depth, structural events not identifiable by traditional CG, normal samples, AML enrichment, paired RNA-seq, and single-cell DNA-seq.
Methods:
Baseline bone marrow (BM) samples from 10 AML patients underwent evaluation using standard clinical CG and targeted next generation seq tests. Additionally, samples were extensively characterized using: 200X WGS (bulk and enriched BM), 50X germline WGS (buccal swab and remission blood), 50X RNA-seq, targeted error-corrected (EC) DNA-seq, and single-cell DNA-seq with oligonucleotide-conjugated antibodies (scDNA-seq+ab).
Results:
The AML patients analyzed had a mean age of 71 (30-81) and a range of BM blast percentage by clinical flow cytometry (0.4-33%). Standard clinical assessment revealed 14 CG abnormalities and 20 mutations across all patients. 50X WGS of the bulk BM utilizing previously published conditions (PMID:33704937) successfully identified 89% of the alterations. Increasing the WGS depth to 100X recovered 2 additional abnormalities, increasing the capture rate to 94%.
Enrichment of AML using the primary cell surface marker prior to WGS achieved a 94% capture rate at only 50X depth, with a depth of only 10X required for the CG abnormalities. Integration of 50X WGS on buccal swab samples reduced the large number of structural variant calls and private mutations without the need for aggressive filtering conditions required for tumor-only samples. However, this also resulted in the removal of a few true mutations due to buccal contamination with immune cells. 50X WGS on remission blood samples from 2 patients mitigated this problem.
Next, we sought to determine what additional information WGS could provide that was missed by standard CG (>5Mb) and seq (>5% variant allele fraction, VAF) analyses. Firstly, 3 of the deletions reported by CG were found to be complex translocation events, resulting in different losses and gains than anticipated. Most importantly, decreasing the CNV size threshold (<5Mb) and utilizing SNP data to identify copy neutral loss of heterozygosity (LOH) identified 7 new events in bulk BM and 2 new events in enriched BM. This information provided important prognostic information, including 3 newly identified structural alternations in a patient with a CG-reported normal karyotype, multiple small deletions in critical regions (e.g. within 5q, 7q), and LOH which in combination with molecular data revealed a biallelic loss of important genes (including TP53, EZH2).
Targeted EC DNA-seq was used to detect of variants <5% VAF and identified an additional 23 variants beyond clinical capture sequencing. 50X RNA-seq with integrated WGS DNA/RNA calling was able to validate the mutations and translocation fusion genes reported by 50X WGS, while also capturing an additional 4 variants found by EC DNA-seq.
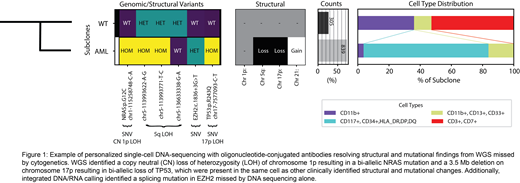
Finally, personalized scDNA-seq+ab provided further resolution of each patient's AML through confirmation of new structural and mutational findings, identification of mutations associated with clonal hematopoiesis versus AML, and definition of AML clonal structure (Figure 1).
Conclusions:
We confirmed that 50X WGS can recapitulate standard clinical CG and mutational profiling in AML patients while providing improved resolution. Deeper seq, AML pre-enrichment, and integrated RNA-seq further aided in identifying low level events. We also demonstrated that WGS can identify additional important structural events missed by CG. Finally, personalized scDNA-seq+ab was able to define clonal architecture important for prognostic and residual disease tracking. Together, these results provide a framework for future integration of WGS into the clinic and personalized patient care.
Sciambi: Mission Bio Inc.: Current Employment. Durruthy-Durruthy: Mission Bio Inc.: Current Employment. Hourigan: Sellas: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal